Background to Clinical Evaluation & Investigation Requirements
The Regulation (EU) 2017/7451 of the European Parliament and of the Council of 5 April 2017 on medical devices – came into effect on 26 May 2021, better known as EU MDR 2017/745 – introduced a set of requirements for conducting clinical evaluation and clinical investigation of medical devices in chapter IV comprising Articles 61 to 80.2
A clinical investigation is defined by EU MDR Article 2(45) as: “any systematic investigation involving one or more human subjects, undertaken to assess the safety or performance of a device.”
EU MDR defines clinical evaluation and investigation requirements based on the international standard ISO 14155: Clinical investigation of medical devices for human subjects — Good clinical practice3 and MEDDEV 2.7/4: Guidelines on clinical investigation.4
Notably, the ISO 14155 standard will be updated every five years to keep up with the latest advancements in medical device development, and the revised ISO/CD 14155 is under development as we speak, which means that the requirements for clinical evaluation and investigation defined by EU MDR were based on the previous versions of ISO 14155 harmonised5 with the ISO 14155:2020 on 14 April 2021.
Unlike its predecessor, the Medical Device Directive (MDD)6 included one sole article – MDD Article 15: Clinical Investigation – on the subject and identified the manufacturer or the authorised presentative as responsible for the clinical investigation; the EU MDR aims to define the roles and responsibilities of those conducting the clinical investigation.
Sponsor of the Clinical Investigation, Perspectives
In contrast to MDD, EU MDR introduces the term sponsor similar to ISO 14155, defined in Article 2(49), as: “any individual, company, institution or organisation which takes responsibility for the initiation, for the management and setting up of the financing of the clinical investigation.”
In comparison, ISO 14155:2020 defines (3.49) the sponsor as: “individual, company, institution or organization taking responsibility and liability for the initiation and management of a clinical investigation (3.8), and arranging the financial setup. Note 1 to entry: “When an investigator (3.30) initiates, implements and takes full responsibility for the clinical investigation, the investigator also assumes the role of the sponsor and is identified as the sponsor-investigator.”
As seen from the above definitions, the idea of a sponsor is quite similar.
The sponsor of the clinical investigation is responsible for meeting MDR clinical study-related requirements, whether it is a company, any institution, or individual investigator-initiated clinical studies.
Article 2(54) defines an investigator as: “an individual responsible for the conduct of a clinical investigation at a clinical investigation site.”
Please note that according to EU MDR, sponsors have responsibilities to ensure regulatory requirements relating to quality management system, risk management, post-market surveillance system and post-market clinical follow-up in addition to clinical evaluation and investigation even though we shall concentrate on the latter.
Sponsor Obligations under EU MDR 2017/745
In addition to introducing a Person Responsible for Regulatory Compliance – the infamous PRRC relevant to all manufacturers of medical devices – in Article 15 of EU MDR, chapter VI introduces a set of obligations for sponsors when it comes to clinical investigation.
Above all, the sponsor is responsible for assessing and determining the correct regulatory pathway for clinical investigation, which should be documented.7
For convenience, please find the sponsor obligations summarised in the table below:
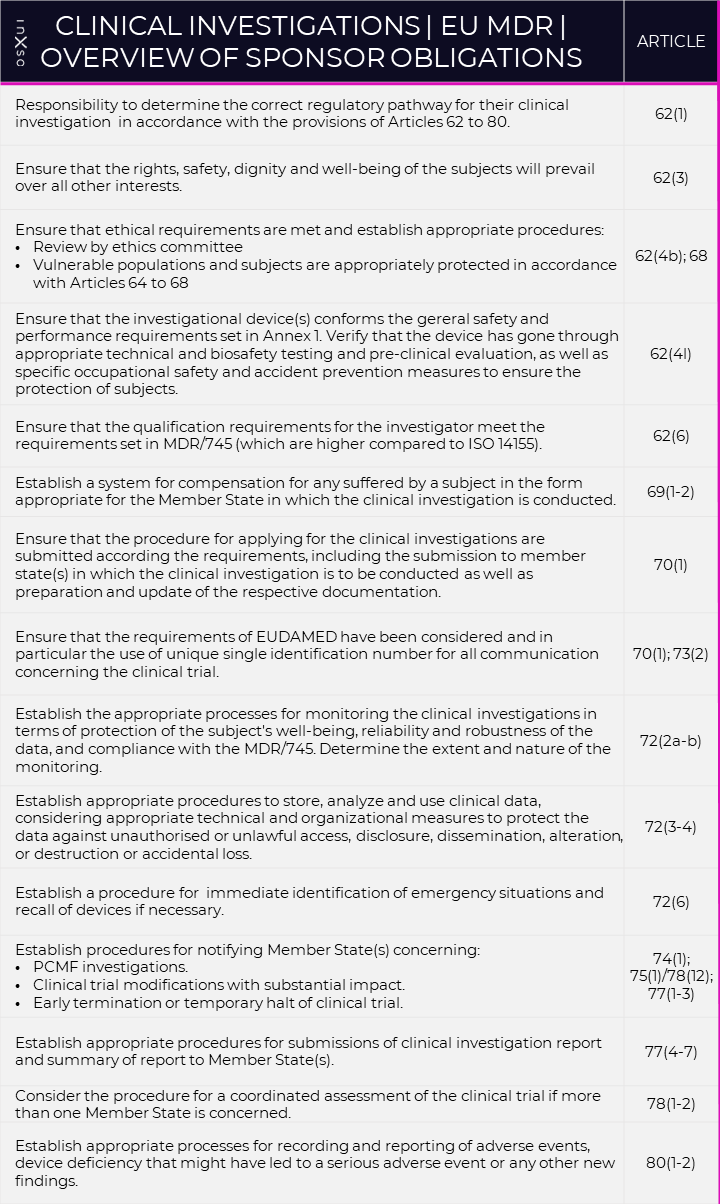
Table 1. Overview of Sponsor Obligations, first published in January 2022 (inXso).
Legal Representatives under EU MDR 2017/745
One of the most important new requirements concerns naming a legal representative for non-EU sponsors conducting a clinical trial in the EU.
According to Article 62(2), the legal presentative is responsible for ensuring compliance with the sponsor’s obligations: “Where the sponsor of a clinical investigation is not established in the Union, that sponsor shall ensure that a natural or legal person is established in the Union as its legal representative. Such legal representative shall be responsible for ensuring compliance with the sponsor’s obligations pursuant to this Regulation, and shall be the addressee for all communications with the sponsor provided for in this Regulation. Any communication with that legal representative shall be deemed to be a communication with the sponsor.”
However, the EU MDR has left the possibility for exemption, as a Member State may waive the requirement for a legal representative in cases where the clinical investigation is conducted only in its territory.
Takeaways
EU MDR has brought various positive changes in conducting medical device clinical trials in contrast to previous quite outdated MDD as well as the roles of those involved.
The transition period has been long since the European Database on Medical Devices (EUDAMED)8 has not been fully functional. The latest version of EUDAMED 2.12 has been released on 20 September 2023.
Clinical investigation will evolve in the future as international standards, as well as the regulatory framework, keep developing with the science.
