Catalysing Breakthroughs in Deep Tech: The EIC Fund Vision and Structure
The EIC Fund, acting as the investment arm of the European Innovation Council, is uniquely positioned to address the financing void that hinders the progress of deep tech startups, especially in the health and medical domains.
Established as a capital fund under private law, with the European Commission as a shareholder, it boasts a budget exceeding €10 billion, signifying Europe’s commitment to leading deep tech innovation.
Operational Dynamics and Impact
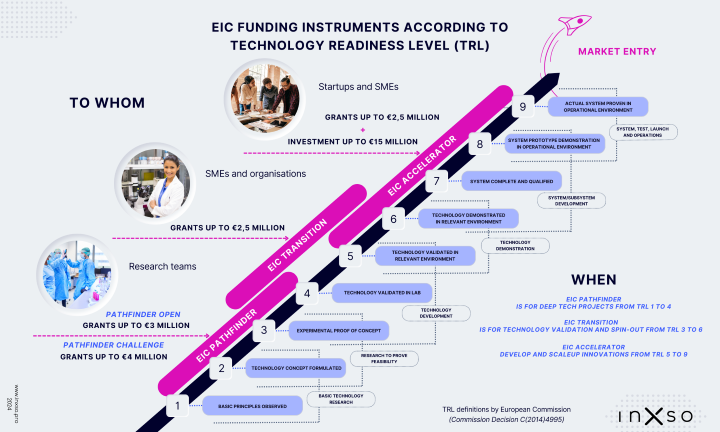
Targeting companies with market-creating potential yet considered too high-risk by conventional investment entities, the EIC Fund provides patient capital, focusing on acquiring minority ownership stakes with investments ranging from €0.5 million to €15 million per entity.
EIC Fund’s strategic approach is designed to nurture potential unicorns, emphasising transformative innovations.
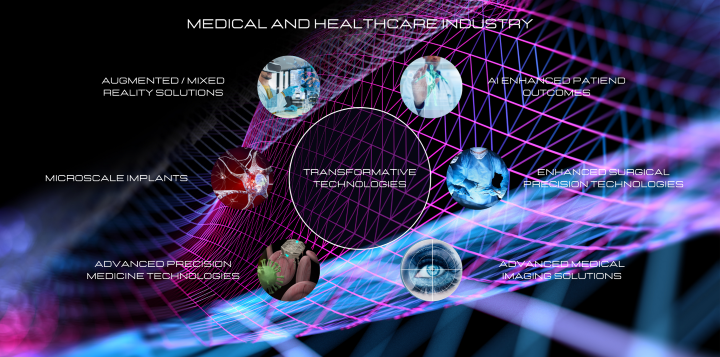
Transformative technologies such as microscale implants, biocompatible devices, advanced medical imaging technologies, wearable biosensor devices, and pioneering treatments for chronic conditions are emerging in the medical and healthcare industry.
A Guide to the EIC Accelerator 2024 for Pioneering SMEs and Startups
In the rapidly evolving landscape of global health and technological innovation, the European Innovation Council (EIC) stands at the forefront, catalysing the transformation of groundbreaking ideas into market-ready solutions.
The EIC Accelerator Programme for 2024 represents a pivotal opportunity for small and medium-sized enterprises (SMEs), startups, and innovators poised to redefine industry norms and address complex challenges.
This programme is not merely a funding opportunity but a launchpad for visionaries determined to create a profound impact with their innovations.
Your Gateway to the EIC Accelerator in 2024
The EIC Accelerator presents a dual pathway for applicants, offering unparalleled flexibility and opportunity:
EIC Accelerator Open Call: Open to proposals across any field of technology or application without predefined thematic priorities.
This call encourages a blend of grant and equity, with a grant cap of €2.5M and an investment component ranging from €0.5M to €15M. With a total budget of €375M for the Open Call, the programme invites single SMEs, startups, and individuals with visions of establishing a startup/SME within EU Member States to apply.
EIC Accelerator Challenges: Besides the Open Call, the EIC Accelerator introduces six challenges in emerging and strategic technologies.
Both the Open Call and the Challenge Calls share identical eligibility criteria and conditions, with a combined indicative budget of €300M allocated specifically for the challenges.
These challenges cover diverse areas, from Human Centric Generative AI to renewable energy sources, reflecting the EIC’s strategic focus on addressing critical technological and societal needs.

The EIC Accelerator’s Comprehensive Support
Beyond financial support, the EIC Accelerator offers a suite of acceleration services designed to propel companies to new heights. Selected companies benefit from business coaching, mentoring, and additional acceleration services, connecting them with investors, corporations, and other entrepreneurs.
This holistic approach ensures innovative projects’ viability and thriving success in European and international markets.
The Path to EIC Accelerator Success
Securing EIC Accelerator funding requires more than just an innovative idea.
It demands a compelling presentation of the innovation’s potential to revolutionise markets or create new ones, a clear international growth ambition, in-depth market knowledge, and a detailed business and financing plan.
The journey begins with submitting a full proposal, followed by an invitation to the highest-ranked proposals for a pitch presentation in Brussels, providing a unique opportunity to showcase your innovation before an expert jury.
About the European Innovation Council (EIC) Accelerator
The EIC Accelerator stands as a testament to the EU’s dedication to supporting high-risk, high-potential SMEs and innovators.
By offering funding, optional equity, and an extensive array of business acceleration services, the EIC Accelerator not only nurtures innovation but also ensures its successful market introduction.
This initiative is more than a funding programme; it is a partnership with innovators, empowering them to achieve unprecedented success and visibility in European and international markets.
In the realm of health technology and beyond, the EIC Accelerator Programme for 2024 is your portal to making a lasting impact. With its comprehensive support system, significant funding opportunities, and a focus on groundbreaking innovations, the EIC Accelerator is ready to transform your visionary projects into reality, driving economic growth and societal well-being.
As you consider applying to the EIC Accelerator, remember that you’re not just applying for funding; you’re stepping into a partnership that values innovation, growth, and global impact.
Accelerate Your Innovation Journey with inXso
As a recognised partner of the EIC, inXso is dedicated to propelling EIC-funded innovators toward success.
Our collaboration with the EIC grants beneficiaries exclusive access to a suite of enhancement services, including expert coaching, mentorship, and strategic networking, all aimed at accelerating business growth.
Explore inXso’s support for your innovation journey: Access our services now.